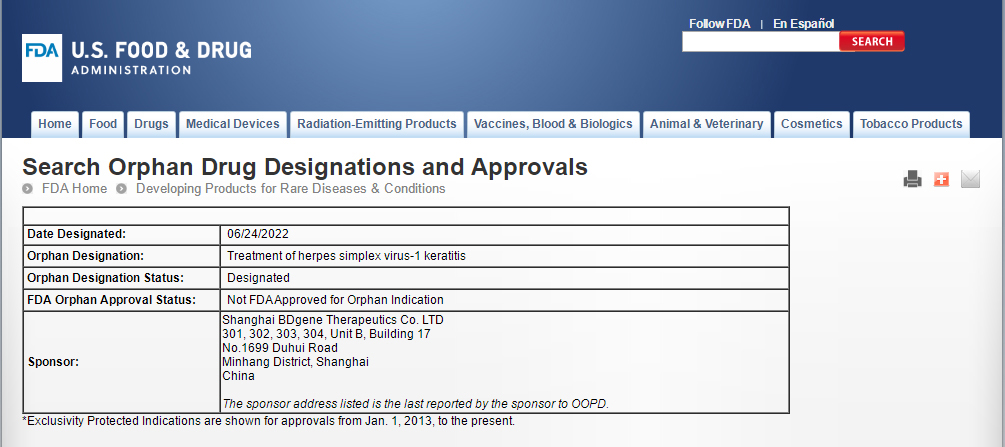
BDgene has completed the effectiveness study and preliminary safety study of CRISPR technology in the treatment of herpesvirus keratitis. The product BD111 has entered the stage of clinical research application for the registration of innovative biological products, and obtained the approval of orphan drugs from FDA on June 24, 2022.
Herpesvirus keratitis, caused by herpes simplex virus (HSV-1), is the most common infectious blinding disease. At present, commonly used antiviral drugs can only inhibit the replication of the virus by interfering with the synthesis of viral DNA. Such drugs can inhibit the replication of HSV-1 DNA, but they can not remove the latent viral genome in the cornea and trigeminal ganglion, so the disease will still occur repeatedly, and even lead to blindness in severe cases.
CRISPR gene editing technology can directly degrade the virus genome and may completely cure the disease. BD111 only needs to be injected once. The drug uses bdgene's original delivery technology VLP to transduce CRISPR, directly target and cut the genome of HSV-1, so as to eliminate the genome of HSV-1 virus and achieve the treatment of herpesvirus keratitis. BD111 is characterized by: (1) delivering cas9 mRNA, the gene enzyme stays in the body for a short time, which can reduce the immune response and reduce the risk of gene editing miss; (2) It edits the viral genome, does not need to change any human genes, and has not detected the off target effect on the human genome.
BD111 has obtained the FDA orphan drug qualification, which will accelerate its clinical trials in the United States and the speed of drug registration and marketing. This will encourage the BDgene team to continue to develop more internationally competitive innovation pipelines, develop more innovative gene therapy products, and provide patients with safe and efficient gene therapy drugs.


