On January 22, 2022, Shanghai BDgene Technology Co., Ltd. announced that important progress has been made in the clinical trial of "anti-neovascular drugs based on IDLV genetic engineering technology for the treatment of age-related macular degeneration",which is a collaboration between Fudan University Eye, Ear, Nose and Throat Hospital (Shanghai Ent Hospital) and Shanghai BDgene Technology Co., LTD.
On January 20, 2022, the patient, Mr. Ma (67 years old), was re-examined in the hospital after receiving gene therapy for age-related macular degeneration for one month.

Professor Xu makes a return visit to Mr. Ma
After several follow-ups and examinations one month after the treatment, Professor Xu found that the patient's subretinal fluid was partially absorbed and showed other signs of improvement. Visual function also improved, from 20 reading letters before treatment to 27, without any local or systemic side effects.
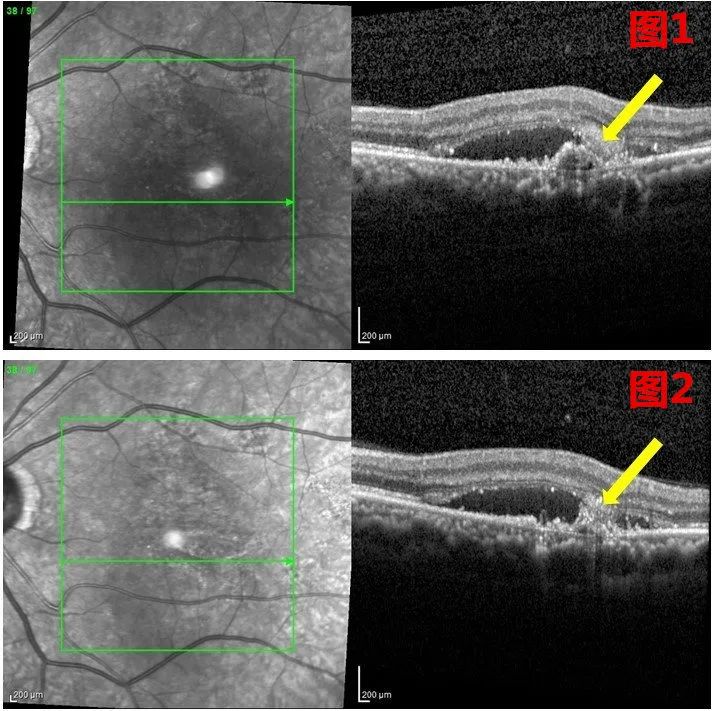
Note: Compared with the 3rd week after treatment (Figure 1), the macular exudation was reduced in the 4th week after treatment (Figure 2), and the cytopathic changes were less than before (yellow arrow).
This means that Professor Xu's team and the BDgene team have made important progress in the genetic diagnosis and treatment of difficult fundus diseases: age-related macular degeneration may achieve safe, effective and long-lasting gene therapy. This provides a new treatment option for patients with refractory age-related macular degeneration in the future, and also provides a useful reference for exploring gene therapy for other difficult eye diseases.
Age-related macular degeneration is a common irreversible blinding eye disease in the elderly. It is characterized by the formation of choroidal neovascularization in the macular region. If not treated in time, it will lead to severe damage to central vision and even blindness due to hemorrhage. The current conventional treatment method is to inject anti-neovascular growth factor into the patient's eye, but it needs to be injected regularly and repeatedly.
In 2018, Mr. Ma was diagnosed with age-related macular degeneration with choroidal neovascularization, and received 16 intraocular injections in four years. As the disease progressed, the interval between his treatments was shortened from the initial 9 months to at least once every 3 months. Because of the severe loss of central vision, his mobility is inconvenient, and he needs to be accompanied by his family every time he goes to the hospital for treatment. For patients like Mr. Ma, it not only increases the financial and spiritual burden of the family, but also worries them that multiple intraocular injections may bring risks such as intraocular hemorrhage and infection.
In order to explore sustainable, safe and effective treatment methods for patients with difficult fundus diseases such as age-related macular degeneration, after detailed preclinical research and strict review by the hospital ethics committee, the team of Professor Xu Gezhi and Professor Hong Jiaxu from Shanghai Ophthalmology Hospital and Shanghai BDgene Professor Cai Yujia's team cooperated and on December 22, 2021, the first patient who entered the clinical trial of the experimental group was treated with anti-neovascular drugs based on IDLV genetic engineering technology.Relevant gene therapy drugs come from GMP-grade cell and gene therapy production workshops and undergo strict production quality control. The research team hopes that through this technology, the body can continuously produce "therapeutic drugs" to achieve "one-time treatment, life-long effective". Now, preliminary results suggest that gene therapy for age-related macular degeneration may be a safe, effective and long-lasting approach.
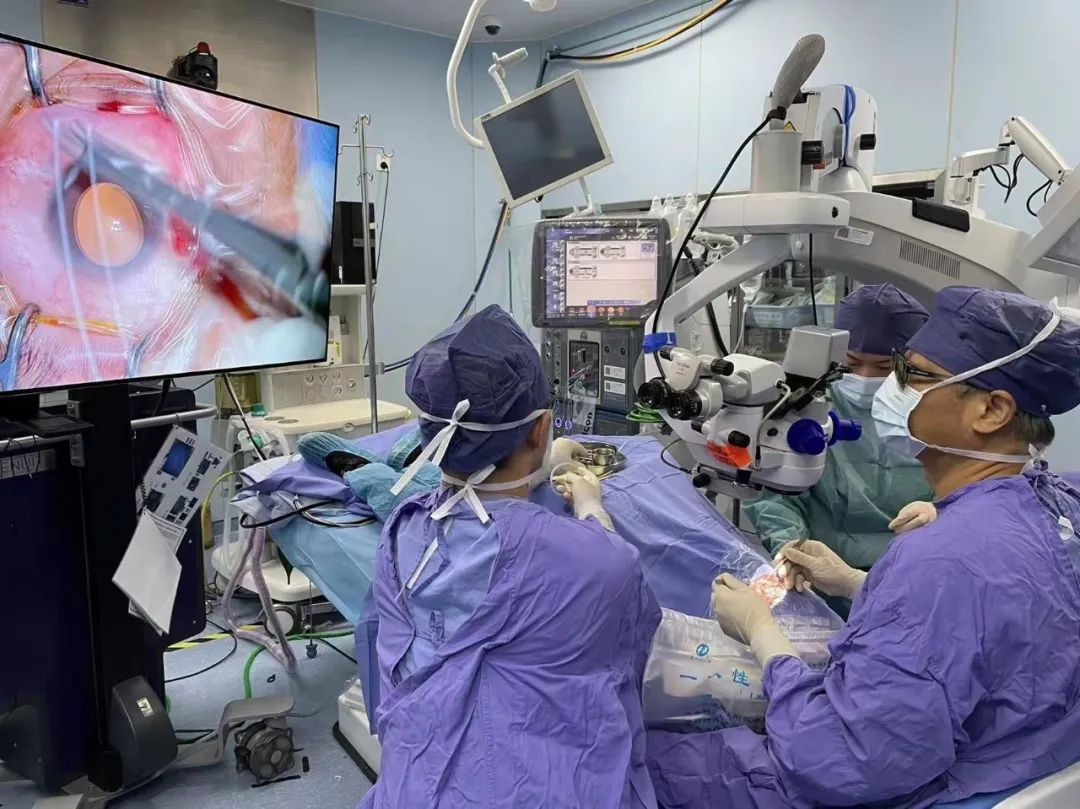
Professor Xu performs surgical treatment for the patient
This is the second gene therapy for difficult eye diseases carried out by the Department of Ophthalmology of Shanghai Ophthalmology Hospital and BDgene. Professor Hong Jiaxu and the BDgene team began to cooperate in exploring the treatment of refractory viral keratitis as early as 2018 and achieved innovative breakthroughs. The relevant results and operating techniques will be published in 2021 in the top international academic journals Nature Biomedical Engineering and Nature Biotechnology. Related clinical application studies have been carried out.


