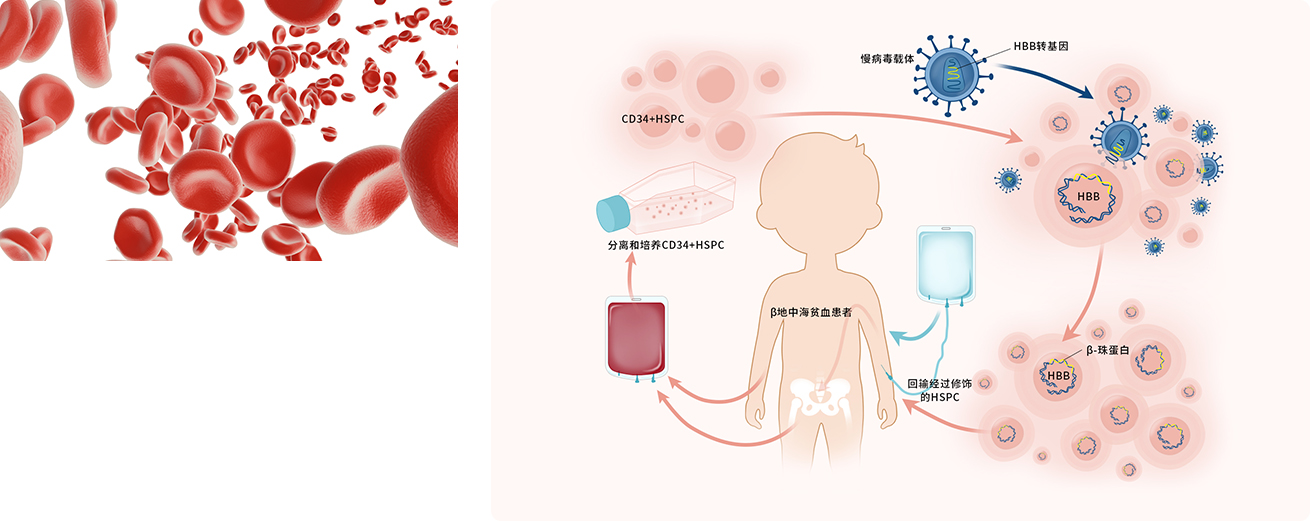
Lentiviral vector transduce autologous CD34+ hematopoietic stem cells to treat transfusion-dependent β-thalassemia, and 2 case of IIT human clinical has been completed. This is the first successful case of treating thalassemia major based on lentiviral vector gene transduction technology in China.

The "thalassaemia" gene therapy developed by BDgene is promising: "One treatment, life-long cure". Firstly, the patient’s hematopoietic stem cells are isolated, activated and cultured in vitro, the correct β-globin gene is compensated to the patient’s hematopoietic stem cells through BDgene's unique lentiviral vector, and then a series of functional and safety tests are performed in vitro , And then return the hematopoietic stem cells with the correct gene to the patient for hematopoietic stem cell transplantation to re-establish and repair the patient’s hematopoietic system.