
On December 30, 2021, Shanghai Bendao Gene Technology Co., Ltd. announced to the public that: The 920th Hospital of the Joint Service Support Force of the Chinese People’s Liberation Army and Shanghai Bendao Gene Technology Co., Ltd. jointly carried out a clinical trial. The experiment is a "Safety and efficacy of lentiviral vector transduction autologous CD34+ hematopoietic stem cells in the treatment of transfusion-dependent β -thalassemia", this clinical trial has achieved preliminary results. This is the first report of a successful case of treatment of β-thalassemia based on lentiviral vector gene transfer technology in China.

Patient Pingping (pseudonym) was diagnosed with severe (β0/β0) thalassemia from genetic testing at the age of 1, and for the past ten years, she needed blood transfusion every 20 days or so to maintain the hemoglobin concentration in the body. After the patient entered the experimental group, after nearly a month of concerted collaboration between the doctors of the 920 Hospital and the gene team, Pingping successfully reinfused the genetically corrected autologous hematopoietic stem cell preparation, and hematopoietic reconstruction was successful. Immune cells and platelets have returned to normal levels. After 3 months of observation and records, Pingping's hemoglobin level is gradually improving, she has initially got rid of blood transfusion, and is continuing to recover.
In recent years, my country's biopharmaceutical industry has developed rapidly, and a number of excellent gene therapy companies have emerged. They are working hard to overcome thalassemia. Shanghai BDgene founded by Professor Cai Yujia is one of them.
Professor Cai Yujia has been devoted to the research work of gene therapy and vector delivery technology for more than 10 years. He founded Shanghai BDgene Technology Co., Ltd. in 2018. The company's thalassaemia gene therapy with independent intellectual property rights can hopefully achieve "one-time cure".
BDgene thalassaemia treatment principle: first isolate the patient's hematopoietic stem cells, activate and culture them in vitro, and introduce the correct β-globin gene into the patient's hematopoietic stem cells through BDgene's unique lentiviral vector. Then, after functional and safety tests are performed in vitro, hematopoietic stem cells with the correct genes are returned to the patient's body to rebuild and repair the patient's hematopoietic system. The schematic diagram is as follows:
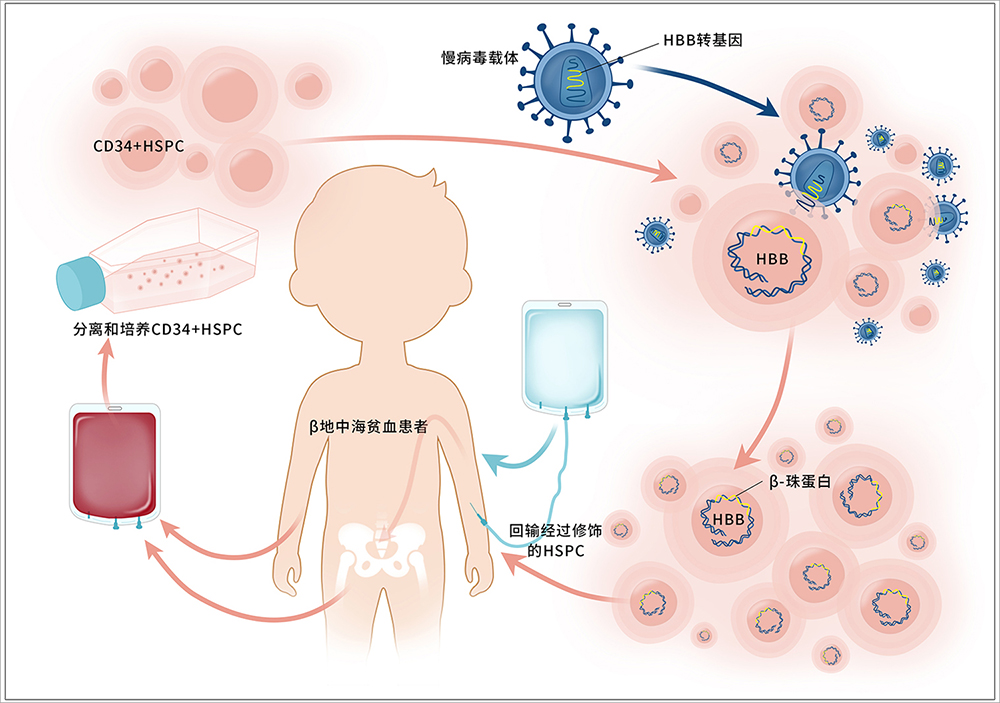
At present, BDgene is actively advancing its IND application for β-thalassemia gene therapy based on lentiviral vectors, bringing this treatment method to the market as soon as possible, and making gene therapy drugs that everyone can consume. Let more thalassemia patients receive effective treatment as soon as possible.
BDgene is a Biotech company dedicated to the development of gene therapy drugs based on innovative vector technology. In recent years, the company's core technologies have been published in top journals such as Nature Biotechnology, Nature Biomedical Engineering, etc., and have received extensive attention from international counterparts. Since its establishment in 2018, the company has made breakthroughs in multiple clinical studies of ophthalmology and hematopoietic system.


